A small biotech company that sent its stock valuation soaring after revealing it had developed a drug that could potentially reverse the cognitive decline of Alzheimer's disease - which would be the first of its kind - is now facing allegations of manipulating or falsifying data.
Cassava Sciences, an Austin, Texas-based company, announced last year that its drug simufilam showed incredible promise in early trials — and it was rewarded with incredible stock growth, reaching a high of $135 per share after spending years below the $5 mark.
But then came the skeptics, and now several experts have pointed to irregularities in published data and even made serious allegations that the company and its associated researchers manipulated data.
The company is now facing an investigation by the Securities and Exchange Commission (SEC), and some of its trial data has either been retracted or flagged by trade publications as potentially manipulated.
This is the second major controversy surrounding a newly developed Alzheimer's drug in the past 12 months, with Biogen's Aduhelm also facing a wave of scrutiny after it was approved by the Food and Drug Administration (FDA) last year.

Biotech company Cassava is facing multiple allegations that it and associated researchers manipulated data and doctored photos related to trials for its Alzheimer's drug simufilam
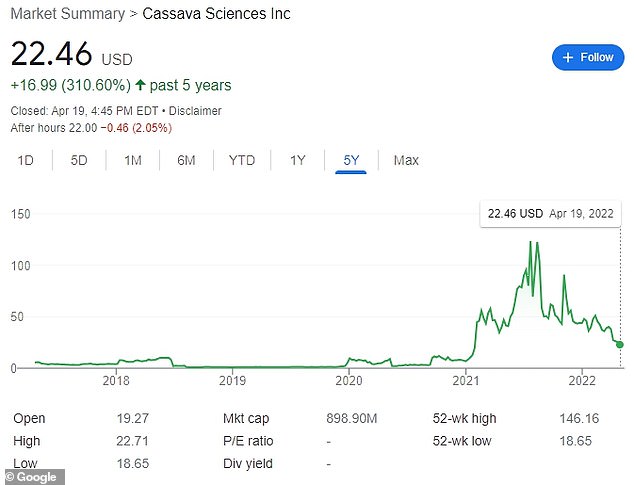
Cassava's share price experienced rapid growth after it released promising data from trials of its Alzheimer's drug. Since then, it has been reeling amid mounting allegations of data manipulation
The allegations focus on two researchers, Hoau-Yan Wang of CUNY in New York City, and Cassava's own Lindsay Burns, who previously published two studies on the brain.
Late last year, the Journal of Neuroscience issued a “statement of concern” regarding data published by the two researchers in late 2021, reports Withdrawal clock.
This means that the newspaper's editors have doubts about the accuracy of the published data.
Last month, another study authored by the pair that is crucial to Cassava's findings was met with an "expression of concern." Neurobiology of aging.
Last year, New York City-based law firm Labaton Sucharow filed a lawsuit Citizens' initiative to the FDA and said it had "serious concerns about the quality and integrity of the laboratory-based studies surrounding this drug candidate and support claims about its effectiveness," Retraction Watch reports.
The law firm represents investors who had shorted the company's shares and would benefit financially from a decline in the company's share price.
PLoS One, a journal that has been published, is just weeks late criticized by many experts for not particularly rigorous peer review process, five papers by Wang retracted.
The New York Times reports that CUNY has opened an investigation into Wang that is ongoing.
The Wall Street Journal reported in November last year, before concerns were first raised, that the SEC had opened an investigation into the company for potentially defrauding investors by using manipulated litigation data to inflate its stock price.
Cassava was funded by the National Institutes of Health to develop the drug and is now facing investigation by that agency as well, the Times reports.
The drug was apparently able to reverse the cognitive decline associated with Alzheimer's in two-thirds of patients. No other drug on the market can do this.
Only one drug may have the ability to even slow cognitive decline, Biogen's Aduhelm, whose effectiveness is also questioned by many, was rejected by Medicare in the US and its developers were also accused of inappropriate data practices.
Experts noted that some fluctuations in biomarkers reported in the studies did not make sense, casting doubt on the study's results.
“This type of discrepancy really raises questions about the rigor as well as the reliability of these results,” said Dr. William Hu, a neurologist at Rutgers University, told the Times.
Another study found that the drug could restore the shape of some proteins in the brain, which Dr. Thomas Südhof, a neuroscientist at Stanford University, said "there is simply no way, no reasonable way that this could happen," according to the Times.
Elisabeth Bik, an expert in image manipulation who has also found other studies that have used manipulated photos, reported that she found signs of cassava depictions in her study.
The company's shares have suffered as a result of the allegations. It was at $90.91 per share in early November, before the WSJ report was published, and has since risen to $22.46 by Tuesday's close.

 Suche
Suche
 Mein Konto
Mein Konto

